 The current federal administration has adopted changes to child, adolescent, and adult immunization schedules without the processes and review that have historically accompanied these updates. This included changes to COVID-19 recommendations in spring 2025, rescission of the universal hepatitis B birth dose this past December, and the recent upending of the child/adolescent schedule across vaccine groups. The changes, announced via a January 5, 2026 U.S. Department of Health and Human Services decision memorandum and outlined in an HHS Child Immunization Schedule, are significant.
The current federal administration has adopted changes to child, adolescent, and adult immunization schedules without the processes and review that have historically accompanied these updates. This included changes to COVID-19 recommendations in spring 2025, rescission of the universal hepatitis B birth dose this past December, and the recent upending of the child/adolescent schedule across vaccine groups. The changes, announced via a January 5, 2026 U.S. Department of Health and Human Services decision memorandum and outlined in an HHS Child Immunization Schedule, are significant.
With these updates, the federal government signals a downgrade in the importance of these vaccinations among healthy children and adolescents and their role in infection control and prevention for patients and the population. Vaccinations previously indicated as routine recommendations are now indicated as reserved for those at high-risk and /or indicated as having a lacking clearly favorable risk-benefit calculation across the population (and therefore reserved for shared-clinical-decision-making or SCDM). The table below summarizes the changes introduced in the decision memorandum, including these shifts.
HHS updates to child and adolescent immunization recommendations as of January 5, 2026
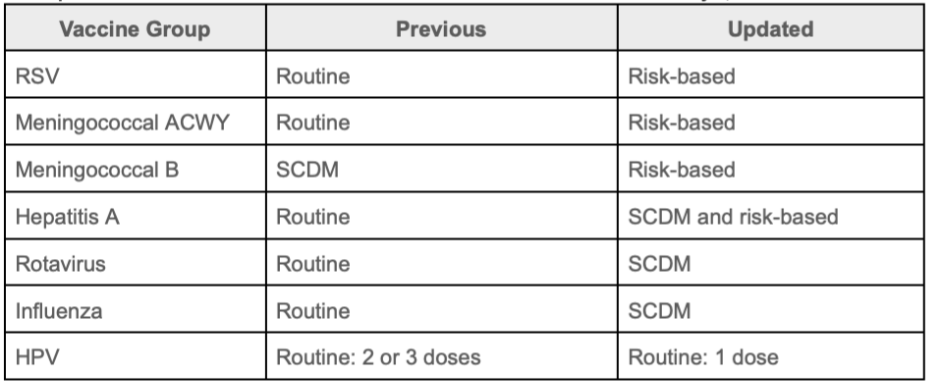
The indications of routine, SCDM, and risk-based recommendations are not new; however, their application and use by the Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention has changed, as they are conflated with the clinical practice of informed consent. With professional medical societies and public health organizations questioning and rejecting changes to federal immunization policies,* keeping track of and understanding the latest recommendations across organizations has become more difficult. Immunize.org summarizes what this means for frontline providers who vaccinate.
What does this mean for the open-source Immunization Calculation Engine (ICE)? HLN remains committed to ensuring the ICE default immunization schedule reflects evidence-based guidelines (see previous comments on evidence-based recommendations and hepatitis B) looking to evidence as published by groups such as the Vaccine Integrity Project and as interpreted by the American Academy of Pediatrics, the American Academy of Family Physicians, as well as other professional associations, to inform ICE’s recommendations.
As we consider the latest changes by the federal government, we continue to grapple with the role of ICE in helping end-users of the software – health care organizations, public health agencies, and consumers– make sense of the fractured immunization recommendation landscape. For example, would it be beneficial to surface both the evidence-based recommendations and the new CDC recommendations within a health IT application?
Technically, ICE is capable of supporting multiple schedules, including the evidence-based default and various alternate configurations. However, whether this is something we should do, how it could be done responsibility, and with what resources remain open questions we look forward to discussing with our ICE Advisory Group.
Sign up to receive notices of new ICE releases via the ICE Announcement listserv. For more information about ICE and/or the ICE Advisory Group, please reach out to us at ice@hln.com.
*The following is a sampling of organizations that have posted public comments on this issue:
Professional Organizations
- American Academy of Pediatrics
- American College of Physicians
- American Medical Association
- Association of Immunization Managers
- Infectious Disease Society of America
- National Association of County and City Health Officials
- National Foundation for Infectious Diseases
Jurisdictional Public Health
- Colorado Department of Public Health & Environment
- Illinois Department of Public Health
- Michigan Department of Health and Human Services
- Minnesota Department of Health
- North Carolina Department of Health and Human Services
- Northeast Public Health Collaborative (Connecticut, Delaware, Maine, Maryland, Massachusetts, New Jersey, New York, Pennsylvania, Rhode Island, Vermont; New York City, Philadelphia)
- Wisconsin Department of Health Services
- West Coast Health Alliance (California, Hawaii, Oregon, Washington)

